Greenhouses gases are atmospheric gases such as carbon dioxide (CO2), methane (CH4), and water vapor (H2O) that absorb and re-radiate heat, which warms the lower atmosphere and Earth’s surface. This process of absorption and re-radiation of heat is called the greenhouse effect. Although greenhouse gases only make up a small percentage of the atmosphere, small changes in the amount of greenhouse gases can greatly alter the strength of the greenhouse effect, which in turn, affects the Earth’s average temperature and climate.
A variety of processes and phenomena can significantly change the amount of greenhouse gases in the atmosphere.
Greenhouse gas molecules. Credit: NASA
Over long timescales, from thousands to millions of years, changes in greenhouse gas concentration can be caused by:
- Volcanism, which releases greenhouse gases, including carbon dioxide and water vapor.
- Increased rates of the weathering of rocks, which through a series of chemical reactions reduces the amount of greenhouse gases in the atmosphere, especially carbon dioxide.
Over shorter timescales, from decades to centuries, changes in greenhouse gas concentration can be caused by:
- Reduction in the amount of permafrost (soil that is frozen all year round), which contains methane (CH4, a greenhouse gas). When temperatures remain cold all year-round organic material decays very slowly, and it remains in the soil. The melting of permafrost, which is happening as global temperatures increase, releases methane. The increasing temperatures also increase rates of decay, which further increases the amount of greenhouse gases in the atmosphere.
- Increases in global temperatures, which increases the amount of evaporation, adding more water vapor to the atmosphere.
- Changes in the amount of biomass and productivity, which alters how carbon cycles and how much carbon dioxide is taken out of the atmosphere by photosynthesizing organisms. Carbon is returned to the atmosphere through respiration, the decay of organic material, and by fires.
Today, changes in the amount of greenhouse gases in the atmosphere are the result of many human activities, including:
- The burning of fossil fuels, which releases carbon dioxide and nitrous oxide (N2O) into the atmosphere.
- Agricultural activities, for example, farm animals, such as cattle and sheep, and the bacteria associated with rice farming, all contribute methane to the atmosphere. Nitrate fertilizers increase nutrient levels which leads to the release of nitrous oxide from the soil, and the running of most farm machinery involves the burning of fossil fuels.
- Deforestation in many areas of the world involves the burning of forests. Fires release carbon dioxide and nitrous oxide into the atmosphere. When forests are cut and the downed trees left to rot, the decay process also releases carbon dioxide to the atmosphere.
- Pollutants & waste from the manufacturing of cement from limestone (which is rich in carbon) and other industrial processes releases carbon dioxide and nitrous oxide into the atmosphere. The accidental leakage of natural gas by the oil and gas industry increases atmospheric methane. Man-made hydrofluorocarbons (HFCs) used for air conditioning and refrigeration are also greenhouse gases.
- Using renewable energy, such as solar and wind, will decrease our dependence on fossil fuels and reduce the increase in the levels of greenhouse gases in the atmosphere.
How do we know that carbon dioxide is rising in the atmosphere? Since the 1960s, scientists have measured atmospheric carbon dioxide concentrations in a variety of locations. Over this time frame carbon dioxide has increased from about 315 parts per million to over 420 parts per million.
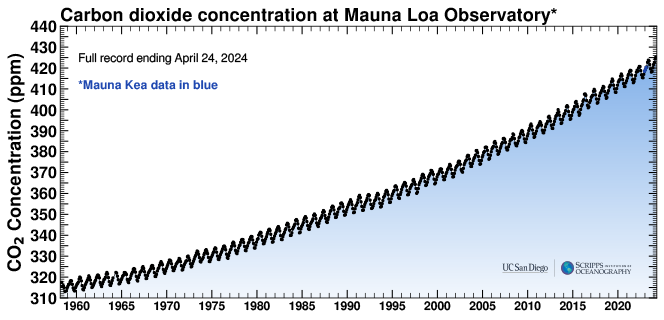
Figure: measured carbon dioxide concentrations. Commonly called the “Keeling Curve”. Credit: https://bluemoon.ucsd.edu/co2_400/mlo_full_record.pdf
Scientists can also extract ancient air trapped in ice from ice cores and measure atmospheric carbon dioxide concentrations going back continuously to 800,000 years ago. Over this time frame, carbon dioxide levels have never been above 300 parts per million. Scientists are now actively attempting to find even older ice.
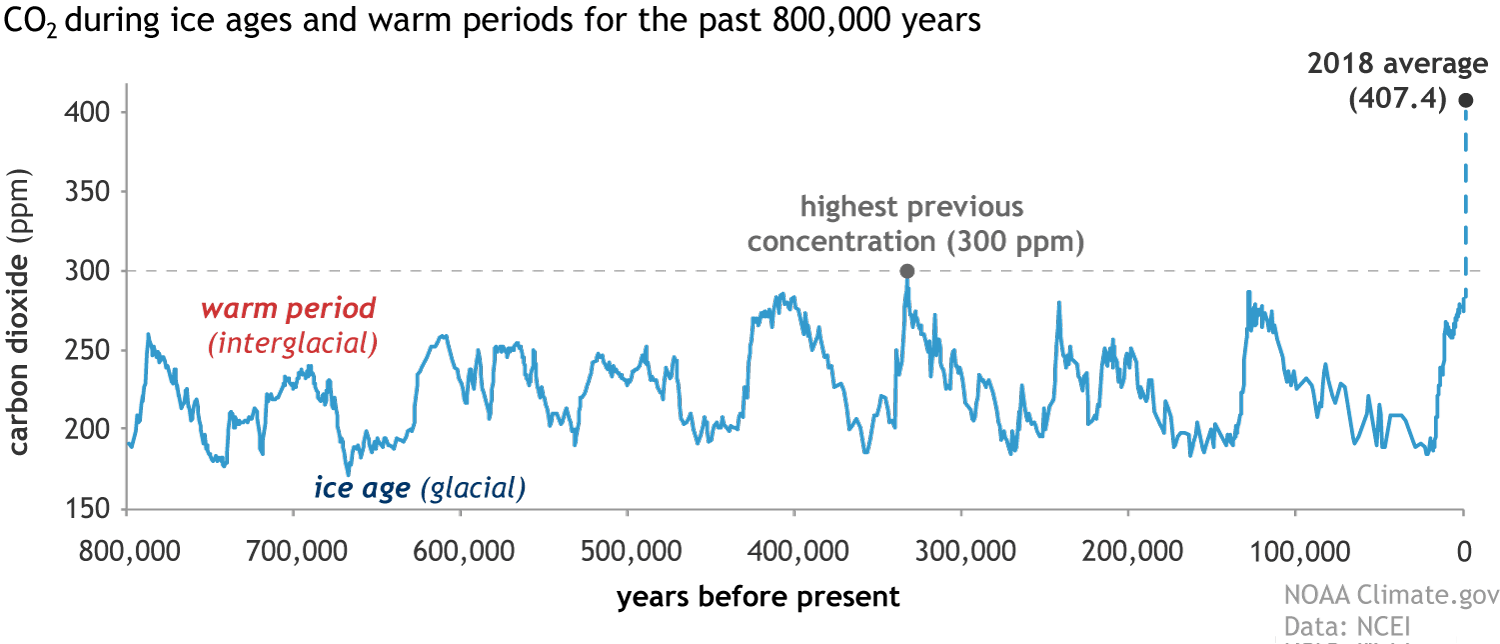
Global atmospheric carbon dioxide concentrations (CO2) for the last 800,000 years. Credit: Climate.gov
Scientists also develop tools to identify and quantify the sources of carbon dioxide to the atmosphere. For example, how do we know the recent rise in atmospheric carbon dioxide concentrations is largely due to fossil fuel burning? One way is to study the chemistry of carbon dioxide, specifically the carbon atoms with different numbers of neutrons (called isotopes). These isotopes can be used to fingerprint sources of carbon dioxide in the atmosphere (learn more in this lesson).
Can you think of additional cause and effect relationships between greenhouse gases and other parts of the Earth system?
Visit the greenhouse effect and re-radiation of heat pages to learn more about how Earth’s energy budget is connected to other global changes.
Investigate
Learn more in these real-world examples, and challenge yourself to construct a model that explains the Earth system relationships.
Links to Learn More
- The Keeling Curve
- How we measure background CO2 levels on Mauna Loa
- What is Warming the World?
- NASA Earth Observatory: Satellite Detects Human Contribution to Atmospheric CO2
- NOAA: How isotopes are used to study atmospheric carbon dioxide sources
- NSF funded project searching for the oldest ice
- Draft teaching exercise on using isotopes to identify sources of carbon dioxide in the atmosphere
