Ocean acidification is driven by increasing carbon dioxide in the atmosphere. Currently about a third of the increase in atmospheric carbon dioxide is absorbed by the ocean. In the ocean, the dissolved carbon dioxide interacts with the water making the water more acidic, thereby reducing its pH. This decrease in pH can impact the growth and survival of marine organisms which alters ecosystems.
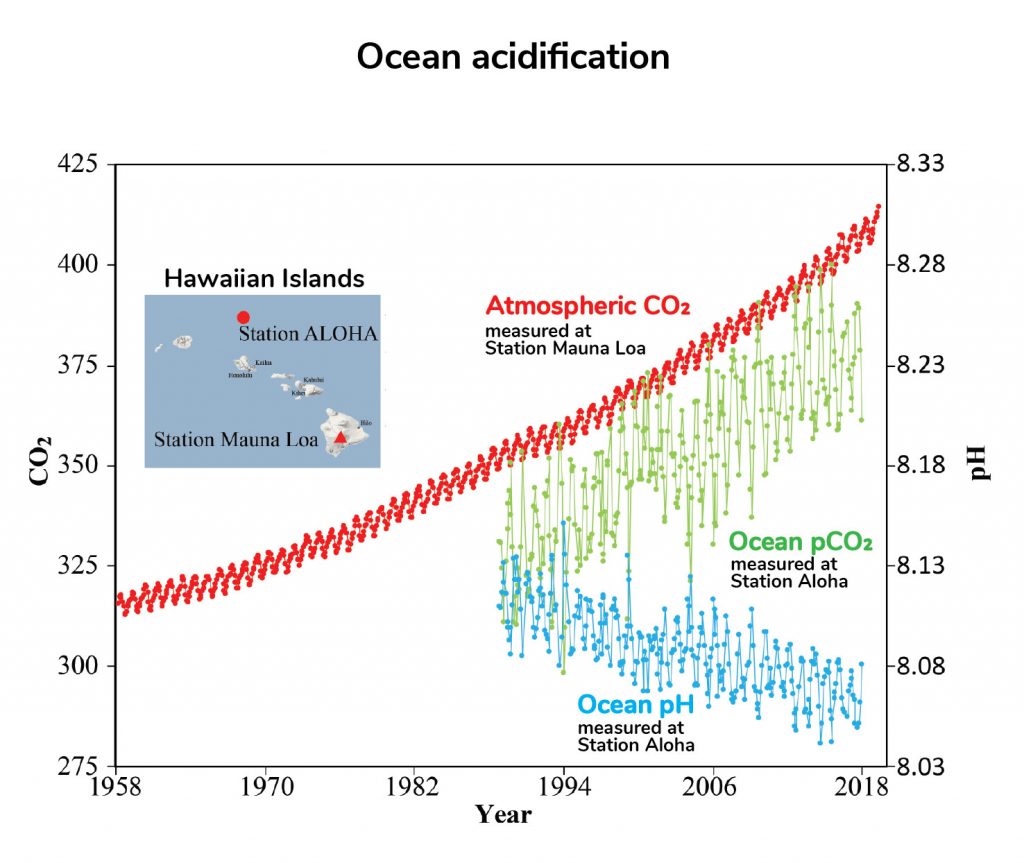
As human activities have increased CO2 levels in our atmosphere (red line), about a third of that CO2 has been absorbed by the ocean (green line), and ocean pH has decreased (blue line). Adapted from NOAA.
Various human activities and environmental phenomena can influence ocean pH, including:
- The burning of fossil fuels, agricultural activities, and deforestation, which increase the amount of CO2 in the atmosphere. Today, oceans are acidifying due to these human activities.Slow, long-term changes in the carbon cycle can influence ocean acidity over thousands to millions of years. For example, a long-term increase in volcanic activity will increase the amount of CO2 in the atmosphere and therefore increase the acidity of the ocean.
- Changes in ocean circulation patterns can alter the pH of the surface waters. For example, upwelling brings deep acidic water to the surface of the ocean. Deep waters are more acidic than surface waters because the CO2 released by deep-sea organisms as they respire is not consumed by photosynthesis, due to the lack of sunlight in the deep.
- Ocean temperature, because the amount of CO2 water absorbs decreases as water temperature increases.
Ocean acidification affects various Earth system processes and phenomena, including:
- Changing biomass and productivity, which can alter species interactions. Lowering the pH of ocean water can cause physiological stress for many organisms, especially those that make calcium carbonate (CaCO3) shells or skeletons, such as corals, making it more difficult for those species to survive and reproduce.
- The evolution of life cycles and traits, as individuals in populations that are more resilient to acidic water are more likely to survive and reproduce.
- Species ranges, as certain areas experience more acidification than others due to ocean circulation patterns.
- Sedimentation in the ocean. There are two types of sedimentary grains, those made for calcium carbonate and those made from other minerals. For example, many white sand beaches are produced by the breaking down of animals with calcium carbonate hard parts, such as coral skeletons and clams and snails shells, and by the formation of carbonate minerals that form in the ocean water. The lowering the pH of the oceans will reduce the amount of calcium carbonate sediments in coastal environments.
Can you think of additional cause and effect relationships between ocean acidification and other parts of the Earth system?
Visit the greenhouse gases, temperature, and burning of fossil fuels pages to explore more connections between the carbon cycle and other global changes.
Investigate
Learn more in these real-world examples, and challenge yourself to construct a model that explains the Earth system relationships.