The ozone layer is located between 10-31 miles above the Earth’s surface within a layer of the atmosphere called the stratosphere. Ozone gas in this layer protects life on Earth by absorbing high energy ultraviolet (UV) radiation from the Sun that can cause sunburns, as well as damage genetic material (DNA), that is cause mutations, that can cause diseases such as skin cancer.
Ozone is made of three oxygen atoms (O3). Ozone forms when sunlight breaks apart oxygen molecules (O2) and free oxygen atoms then bond with other O2 molecules forming O3. Ozone is destroyed when it reacts with other molecules, such as nitrogen, hydrogen, chlorine, and bromine.
Human-made chemicals developed in the 1960s called chlorofluorocarbons (CFCs) also destroy ozone. CFCs are used for various products, including air conditioners, aerosol spray cans, and the manufacturing of Styrofoam. In the 1980s scientists learned that these pollutants were depleting the ozone layer because chlorine atoms from CFCs were breaking apart ozone molecules. In 1986 over 70 counties signed the Montreal Protocol to reduce CFC production, and global cooperation and action have allowed the amount of stratospheric ozone to build back up again.
The stratospheric ozone layer is often confused with ozone pollution closer to Earth’s surface in the troposphere. Surface-level ozone is harmful to life and is created by chemical reactions between air pollutants from burning of fossil fuels and other industrial processes.
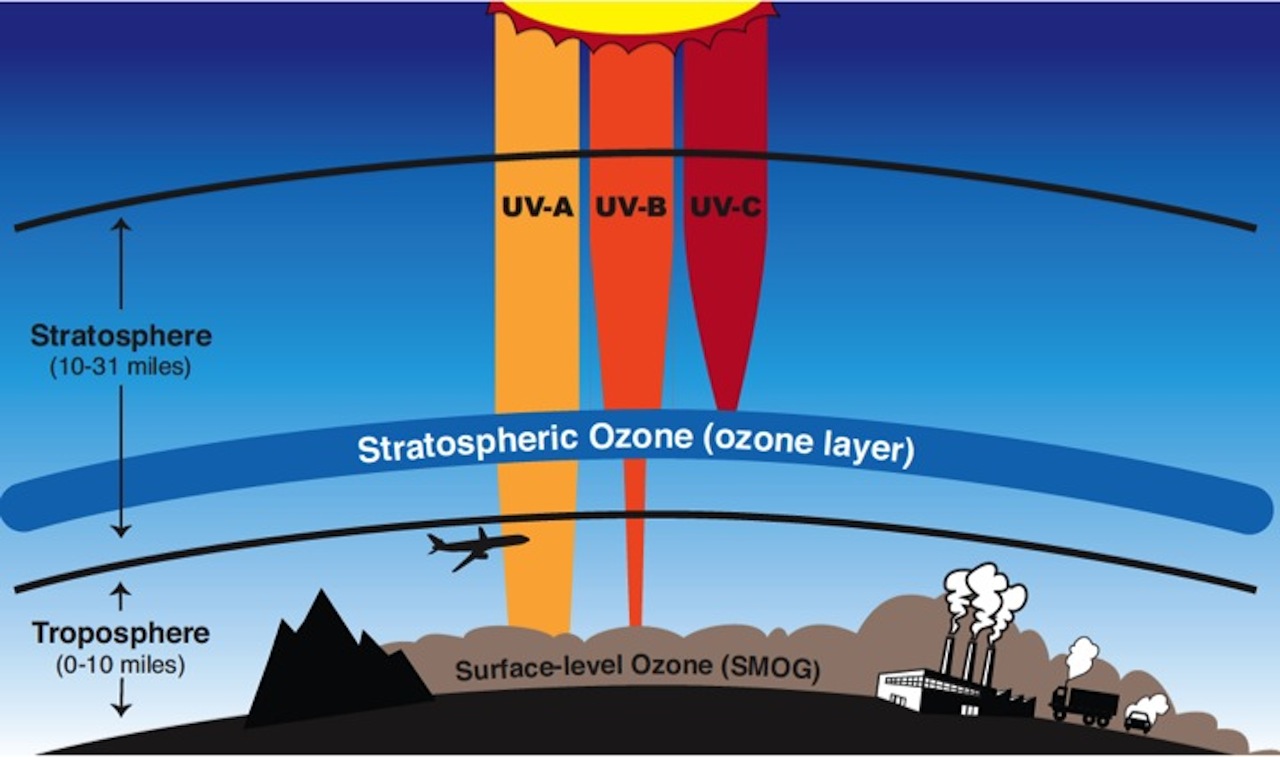
The ozone layer in the stratosphere shields life on Earth from most UV-B and UV-C, the most harmful varieties of ultraviolet radiation. Image Credit: NASA
Can you think of additional cause and effect relationships between the ozone layer and other processes and phenomena in the Earth system?
Visit the airborne particles and greenhouse gases pages to explore more connections between the atmosphere and global change processes and phenomena.
Investigate
Learn more in these real-world examples, and challenge yourself to construct a model that explains the Earth system relationships.
